|
uct Name |
QS-21 |
|
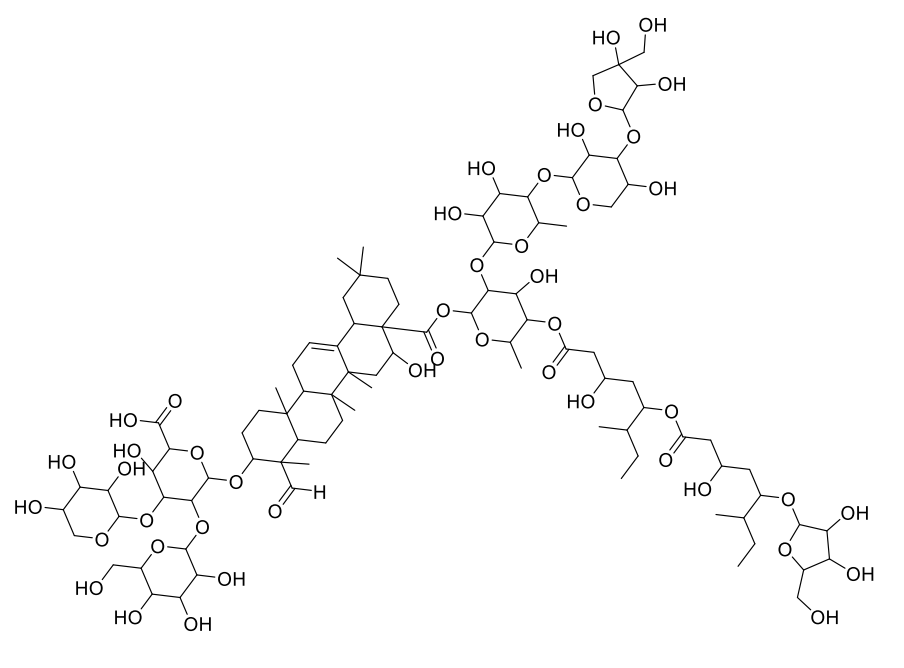
Chemical Name |
|
|
CAS No. |
141256-04-4 |
|
NMPA CDE registration no. |
F20250000075 |
|
USFDA DMF No. |
041805 |
|
Quality grade |
Injection |
|
Quality standards |
Conforms with USP, EP, JP, ChP standards |
|
Molecular Formula |
C92H148O46 |
|
Applications and examples |
QS-21 has been used in SHINGRIX, Mosquirix, Arexvy and other commercially available vaccines developed by GSK. |
|
Product code |
B01001 |
|
Package sizes |
100mg |
|
Storage condition |
Transport: At 2-8°C. Long-term storage: Sealed, protected from light at 2-8°C. Avoid: Exposure to strongly oxidizing agents. Retesting: Annual requalification required. |
QS-21 is an immunostimulatory saponin, capable of stimulating both humoral (Th2) and cell-mediated (Th1) immune responses, and promoting antibody production and antigen-specific T cell responses. It activates dendritic cells (DSs), promotes the production of IgG1 and IgG2a antibodies, and induces the secretion of interferon-γ (IFN-γ). Additionally, QS-21 enhances the function of cytotoxic T lymphocytes (CTLs), thereby combating various infectious diseases and cancers.
However, traditional QS-21 has faced dual constraints of capacity and purity due to limitations in extraction processes. AVT has successfully developed a proprietary separation and purification technology that enables efficient extraction of high-purity QS-21 from Quillaja saponaria. We have also established a stable, reliable quantitative analytical method for routine quality control, separation component analysis, and determination of component content, thereby accelerating the processes for product development and root cause analysis (RCA).
QS-21 Advantages
Leveraging our proprietary separation and purification technology platform, we have simultaneously separated components such as QS-7, QS-17, and QS-18, providing a more comprehensive QS series for vaccine development. In response to the need for higher safety standards in veterinary vaccine applications, we have manufactured high-purity QS-ANM. Relying on our separation and purification technology platform, we now have the capability to supply the full range of QS products and support customized product development, facilitating the vaccine development process.
QS series Product
Leveraging our proprietary separation and purification technology platform, we have simultaneously separated components such as QS-7, QS-17, and QS-18, providing a more comprehensive QS series for vaccine development. In response to the need for higher safety standards in veterinary vaccine applications, we have manufactured high-purity QS-ANM. Relying on our separation and purification technology platform, we now have the capability to supply the full range of QS products and support customized product development, facilitating the vaccine development process.
| Product Name | Product Code | CAS No. | Molecular Formula | Molecular Weight | Availability |
| QS-21 | QS-21-HP | 141256-04-4 | C92H148O46 | 1990.15 | In stock |
| QS-21-MP | |||||
|
QS-7 |
QS-7-HP | 208933-54-4 | C83H130O46 | 1863.89 | In stock |
| QS-7-MP | |||||
| QS-17 | QS-17-HP | N/A | C104H168O55 | 2298.43 | Within 2 months |
| QS-17-MP | |||||
| QS-18 | QS-18-HP | N/A | C98H158O51 | 2152.29 | In stock |
| QS-18-MP | |||||
| QS-ANM | QS-ANM | N/A | N/A | N/A | Within 2 months |
▪ Applications of QS-21 in clinical-stage vaccine programs
Active in 17 clinical indications, including non-small cell lung cancer (NSCLC), herpes zoster, melanoma (phase I clinical trial); Alzheimer’s disease, HIV, tuberculosis, Varicella Zoster, etc.
▪ Applications of QS-21 in approved vaccines
☑ Shingrix® shingles vaccine-GSK
☑ Approved for marketing: in 2017 in the United States and Europe, and in 2020 in China;
☑ Indication: for the prevention of herpes zoster (shingles); not for prevention of primary varicella.
| Category | Component | Content (per Dose) |
| Antigen | Varicella-Zoster Virus Glycoprotein E (gE) | 50 μg |
|
Adjuvant |
Quillaja saponaria Saponin QS-21 | 50 μg |
| 3-O-Desacyl-4'-monophosphoryl lipid A (MPL) | 50 μg | |
| Dioleoylphosphatidylcholine (DOPC) |
1 mg |
|
| Cholesterol | 0.25 mg |
AVT’s QS-21 dual China-U.S. regulatory filing marks a critical milestone in the globalization of China-developed high-grade vaccine adjuvants. We commit to continuous optimization through:
☑ GMP-compliant adjuvant supply systems
☑ Customized development support
☑ End-to-end quality control services



Reviews
There are no reviews yet.